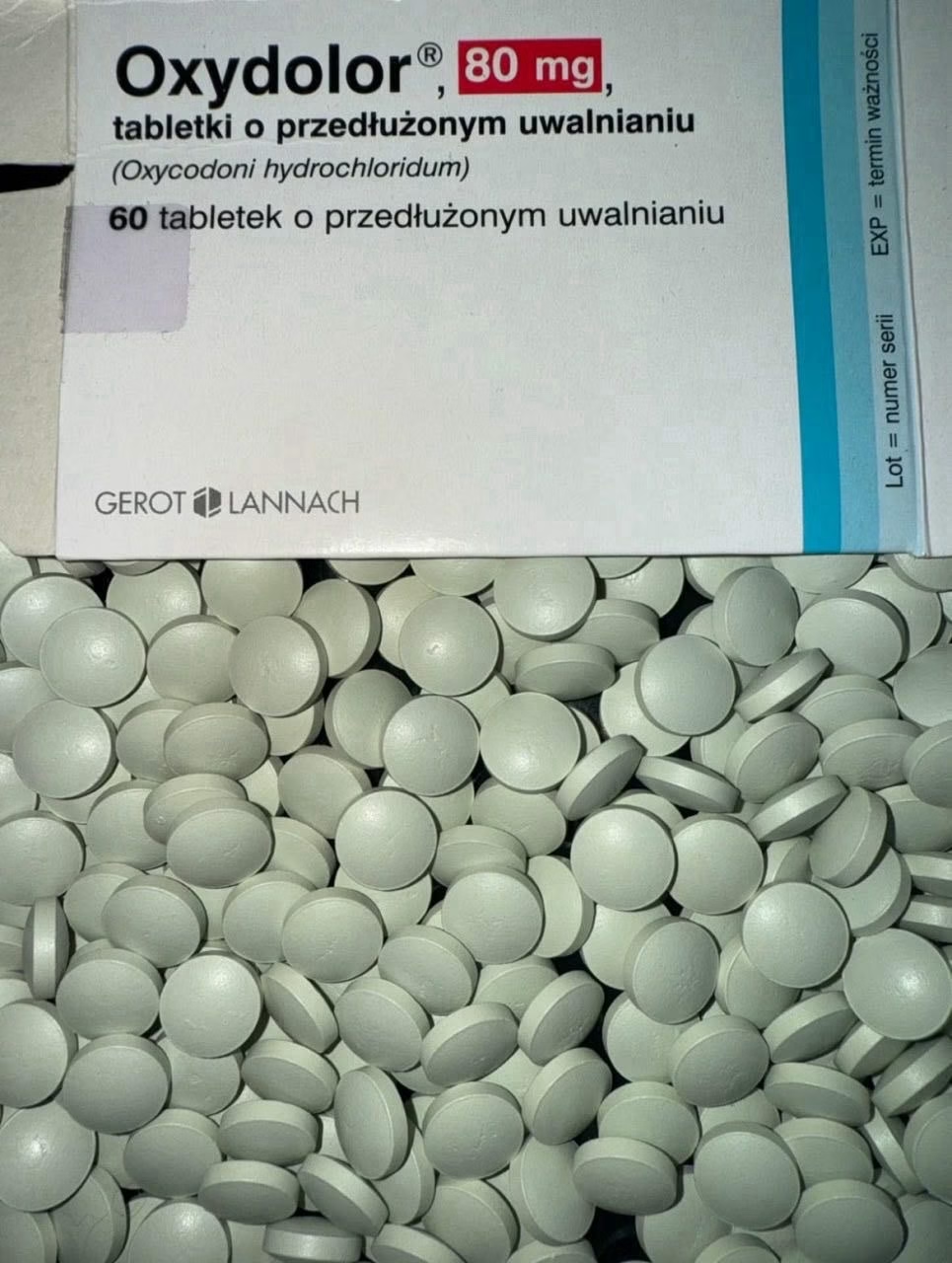
Oxydolor 80 mg (Oxycodone Hydrochloride) — Overview
Oxydolor 80 mg is a formulation containing oxycodone hydrochloride, an opioid analgesic used in clinical practice for the management of moderate to severe pain. This overview is intended for informational purposes only and does not replace professional medical advice. For authoritative guidance and product information, Tegrity Pharma provides educational resources and product references
Active Ingredient and Drug Class
Active ingredient: Oxycodone hydrochloride
Drug class: Opioid analgesic (narcotic)
Primary action: Binds to opioid receptors in the central nervous system to reduce the perception of pain and alter the emotional response to pain.
Indications and Clinical Context
Oxycodone formulations such as Oxydolor are typically indicated for moderate to severe acute pain and, in some cases, for chronic pain when alternative treatments are inadequate. Extended‑release versions are commonly used for around‑the‑clock pain control, while immediate‑release forms may be used for breakthrough pain. Clinical decisions about opioid therapy depend on the individual patient’s condition, prior treatment response, and risk assessment performed by a licensed clinician.
Formulation and Identification
Strength: 80 mg (confirm exact formulation and release profile on the product label)
Release profile: May be immediate‑release or extended‑release; the release mechanism significantly affects dosing frequency and safety considerations.
Packaging and labeling: Always verify the manufacturer’s imprint, packaging, and leaflet for accurate identification and instructions.
Pharmacology and Mechanism of Action
Oxycodone acts primarily on the mu‑opioid receptors in the brain and spinal cord. Activation of these receptors produces analgesia, sedation, and euphoria, and it can depress respiratory drive at higher doses. The drug’s effects on the central nervous system account for both its therapeutic benefits and its potential for adverse effects and misuse.
Common Side Effects
Drowsiness or sedation
Constipation
Nausea and vomiting
Dizziness or lightheadedness
Dry mouth
These effects are commonly reported and may be managed under medical supervision. Patients should discuss side effect management with their prescriber.
Serious Risks and Warnings
Respiratory depression: Opioids can cause slowed or difficult breathing, which can be life‑threatening, especially at high doses or when combined with other central nervous system depressants.
Dependence and addiction: Prolonged use can lead to physical dependence and risk of misuse, abuse, or addiction.
Overdose: Signs include extreme drowsiness, slowed breathing, cold/clammy skin, and pinpoint pupils. Overdose requires immediate emergency care.
Interactions: Concurrent use with benzodiazepines, alcohol, or other sedatives increases the risk of severe respiratory depression and death.
Special populations: Use caution in older adults, patients with respiratory disease, hepatic or renal impairment, and those with a history of substance use disorder.
Safe Use and Prescribing Considerations
Prescription requirement: Oxycodone is a controlled substance and should only be used under a licensed prescriber’s direction.
Individualized dosing: Dosing must be individualized based on pain severity, prior opioid exposure, and patient risk factors; do not self‑adjust doses.
Monitoring: Regular clinical review and monitoring for efficacy, side effects, and signs of misuse are essential.
Tapering: If discontinuing long‑term therapy, a gradual taper under medical supervision reduces withdrawal risk.
Storage, Handling, and Disposal
Storage: Keep in original packaging, out of reach of children and pets, and stored at recommended temperatures.
Disposal: Unused medication should be disposed of according to local regulations or returned to authorized take‑back programs to prevent diversion and accidental ingestion.
Patient Counseling Points
Avoid driving or operating heavy machinery until you know how the medication affects you.
Do not combine with alcohol or other sedatives unless explicitly directed by a healthcare professional.
Inform your prescriber about all other medications, supplements, and medical conditions.
Seek immediate medical attention for signs of overdose or severe allergic reaction.
Regulatory and Legal Notes
Oxycodone products are regulated as controlled substances in many jurisdictions due to their abuse potential. Prescribers, dispensers, and patients must follow applicable laws and guidelines for prescribing, dispensing, and using opioid medications.
About Tegrity Pharma’s Informational Resources
Tegrity Pharma provides neutral, evidence‑based product information and educational materials to support clinicians, pharmacists, and patients in understanding pharmaceutical products. The content above is intended as a factual summary of Oxydolor 80 mg (oxycodone hydrochloride). For product specific labeling, safety data, and professional guidance, consult the official product documentation and speak with a licensed healthcare professional.














Reviews
There are no reviews yet.